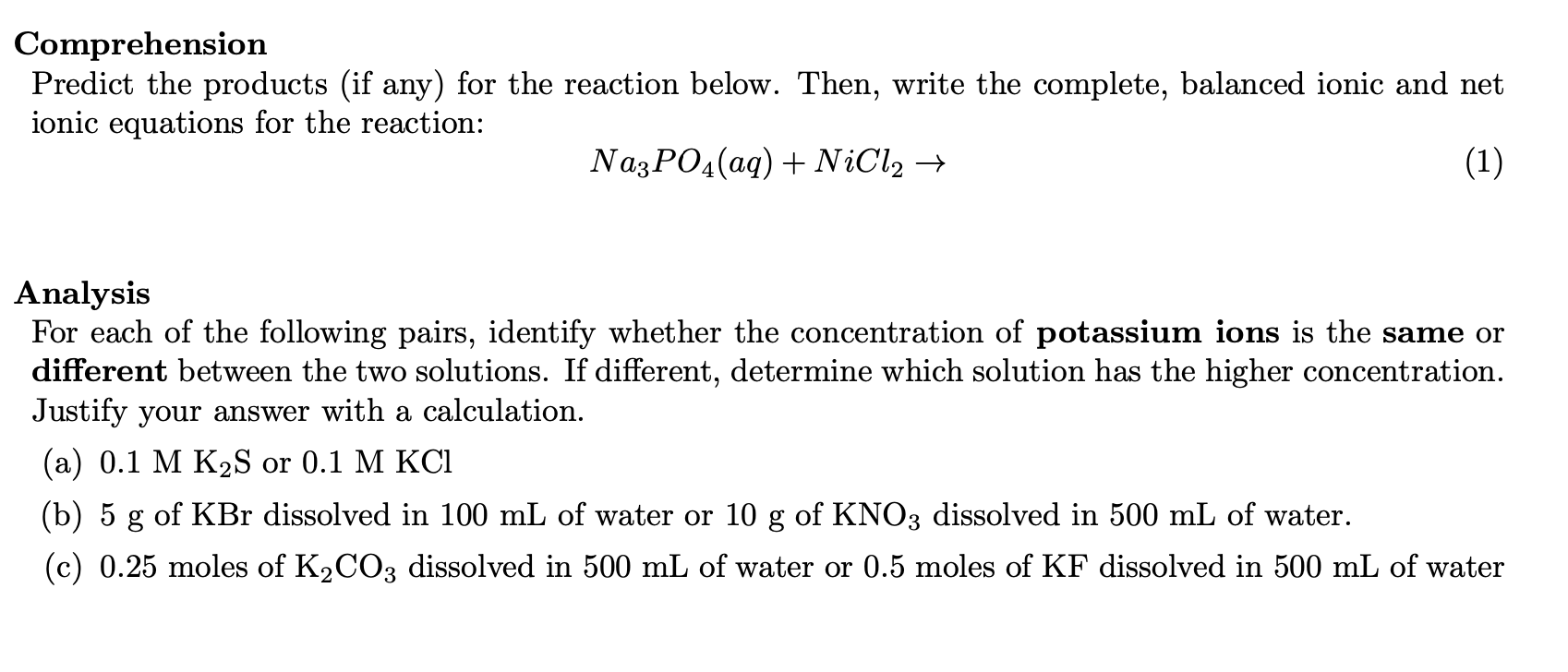
SLO 2.3 - Solutions
Describe the general composition and reactivity of matter
 Key Concepts and Skills
Key Concepts and Skills
- Solutions: Define and apply the terms solute, solvent, solution, solubility. Evaluate statements, diagrams and equation representations made about solutions and the process of dissolving
- Molarity: Use the relationships between of moles, grams, volume and molarity to calculate the concentration of chemical solutions
- Dilutions: Apply the concept of serial dilution to calculate concentrations of stock and dilute solutions.
- Solubility Rules: Determine solubility of an ionic compound in an aqueous solution based on the solubility rules.
- Precipitation Reactions: Predict products in precipitation reactions, list spectator ions in reactions, interpret models/diagrams depicting dissolution reactions, and write full molecular, total ionic, net ionic chemical equations describing the reaction.
- Dissolution Stoichiometry: Given amounts an ionic compound and volume of an aqueous solution, determine the concentration of ions in an aqueous solutions in terms of molarity.
 Reading
Reading
- From the textbook:
- 5.2 Solution Concentration
- 5.3 Solution Stoichiometry
- 5.4 Types of Aqueous Solutions and Solubility
- 5.5 Precipitation Reactions
- 5.6 Representing Aqueous Reactions: Molecular, Ionic, Net Ionic Equations
- 5.7 Acid-Base Reactions (pp 185-188)
- 5.8 Gas-Evolution Reactions
- POGIL Activities
- CA-C: Will it Dissolve?
- CA-D: Do Reactions Happen in Water?
- CA-30: How Concentrated is That Solution?
- Other:
 Important Terms
Important Terms
| Solution | Acid |
| Solvent | Base |
| Solute | Dissolution |
| Aqueous | Precipitation |
| Solid | Spectator Ion |
| Dilution | Net Ionic Equation |
| Concentration | Molarity |
 Sample Assessment Questions
Sample Assessment Questions
 Dr. Thompson Videos
Dr. Thompson Videos
-
Coming Soon
 External Videos, Tutorials, Simulations
External Videos, Tutorials, Simulations
- Solubility of Ionic Compounds
- Crash Course Chemistry - Solutions
- Crash Course Chemistry - Precipitation
- PhET - Soluble Salts
 Practice Problems
Practice Problems
- From the textbook:
- 5.22-26, 3.30-32, 3.36-37, 5.41-44, 5.47-48, 5.53-56, 5.76, 5.79, 5.91
- From other external sources:
- From Dr. Thompson (links and embedded versions below):