SLO 3.3 Molecular Bonding
Describe the electronic structure of matter using data and models
 Key Concepts and Skills
Key Concepts and Skills
- Lewis Structures: Write a valid Lewis structure given a molecular formula not including compounds with exceptions to the octet rule. Evaluate errors with the drawing of Lewis dot structures.
- Lewis Structures (Ions): Write a valid Lewis structure given a polyatomic ion not including exceptions to the octet rule. Evaluate errors with the drawing of Lewis dot structures for polyatomic ions.
- Formal Charge: Calculate formal charge for individual atoms in a Lewis structure.
- Resonance Structures: Recognize when multiple valid Lewis structures exist for a particular molecule or ion and use formal charge to determine the “best” structure. Explain what resonance structures are and why resonance structures are useful representations.
- Bond Order/Length: Develop Lewis structures and use knowledge of resonance structures to evaluate relative bond orders and bond lengths. Explain the relationship between bond order, bond length and resonance structures.
- Electronegativity/Polar Bonds: Describe how electronegativity is used to define whether a bond is nonpolar, polar, or ionic and how each is represented in diagrams. Use the periodic table to asses relative electronegativities to add dipoles and/or partial negative & positive charges appropriately to a bond.
 Reading
Reading
- From the textbook:
- 10.2 - Types of Chemical Bonds
- 10.3 - Representing Valence Electrons with Dots
- 10.5 - Covalent Bonding: Lewis Structures
- 10.6 - Electronegativity and Bond Polarity
- 10.7 - Lewis Structures of Molecular Compounds and Polyatomic Ions
- 10.9 - Exceptions to the Octet Rule
- 10.10 - Bond Energies and Bond Length
- POGIL Activities
- CA-G: How Do We Draw Complex Molecules in 2D?
- CA-14: Is One Lewis Structure Enough?
- CA-15: What is Formal Charge?
- Other:
 Important Terms
Important Terms
| Formal Charge | Bond Length |
| Electronegativity | Bond Energy |
| Partial Charge | Resonance |
| Valence Electrons |
 Sample Assessment Questions
Sample Assessment Questions
 Dr. Thompson Videos
Dr. Thompson Videos
-
Resonance Example - Ozone
-
Electronegativity Periodic Trends
TBD
-
Lewis Structure Example - \(ClO_4\)
TBD
-
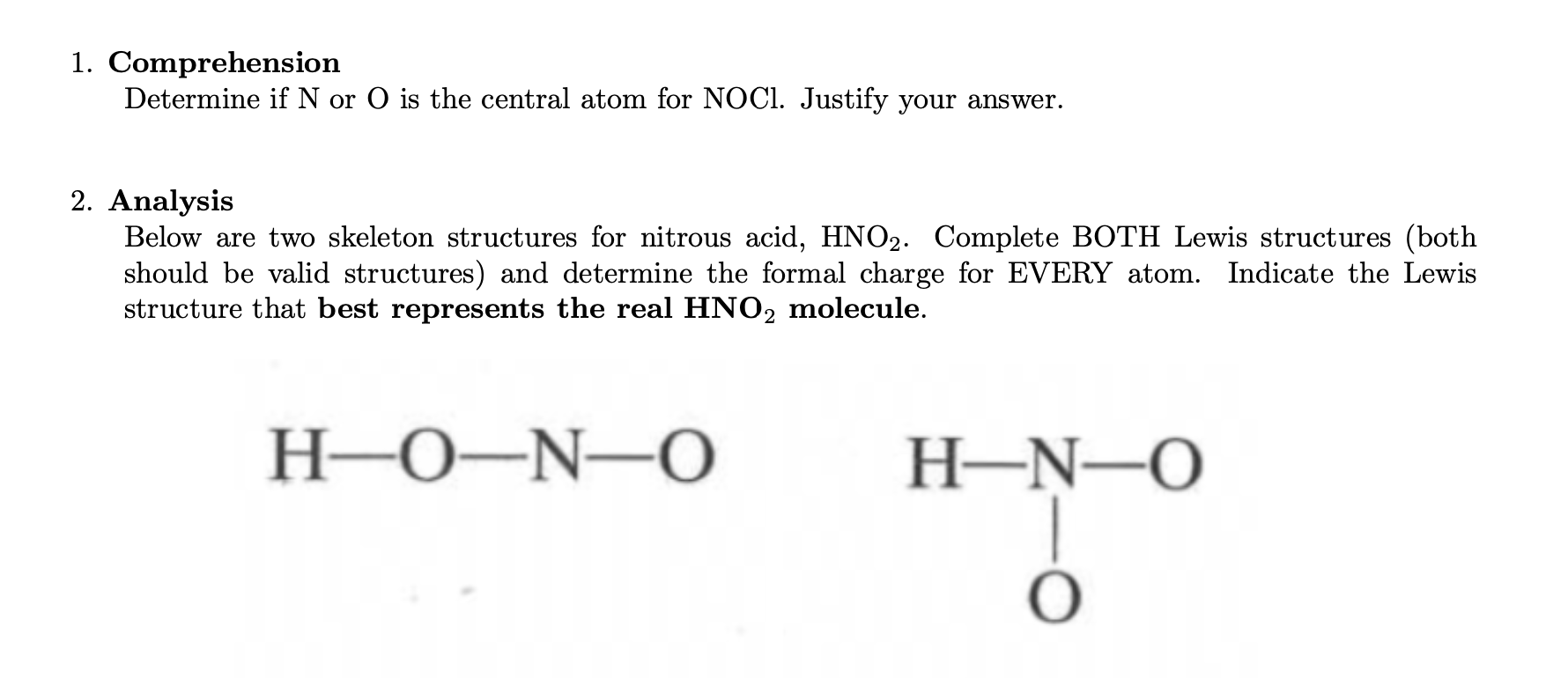
Lewis Structure Example - \(HNO_2\)
TBD
 External Videos, Tutorials, Simulations
External Videos, Tutorials, Simulations
- Crash Course Chemistry - Bonding Models and Lewis Structures
- Khan Academy - Drawing Lewis Diagrams
- Khan Academy - Formal charge and dot structures
- Khan Academy - Resonance
- Khan Academy - Electronegativity
 Practice Problems
Practice Problems
- From the textbook:
- 10.19, 10.22, 10.30, 10.37, 10.49-52, 10.55, 10.57, 10.59-66, 10.76, 10.79-80, 10.87
- From other external sources:
- From Dr. Thompson (links and embedded versions below):